We are interested in designing molecules which test the limits of organic photophysics. Whether we are trying to make a new molecule which absorbs and emits way beyond the bandgap of silicon, or something that is strongly superradiant, our guiding principle is to explore the edges of chemical design and photophysics.
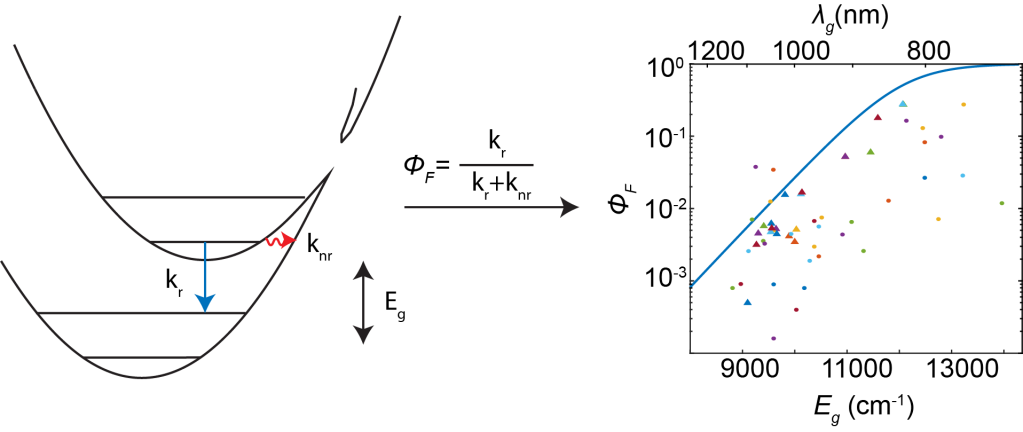
The short-wave infrared (SWIR or NIR II), is the sparse spectral region from 1-2 microns, red of most electronic transitions, yet blue of many infrared active molecular vibrations. Recent research has realized its potential in deep tissue imaging, biometric identification, satellite telemetry for weather and plant cover, and pedestrian imaging for self-driving cars. We aim to understand why organic SWIR emitters are so sparse, through application of fundamental chemical physics principles.
Supramolecular Aggregates Inspired by Quantum Biology
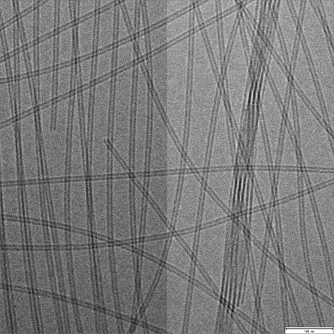
Supramolecular Nanotubes 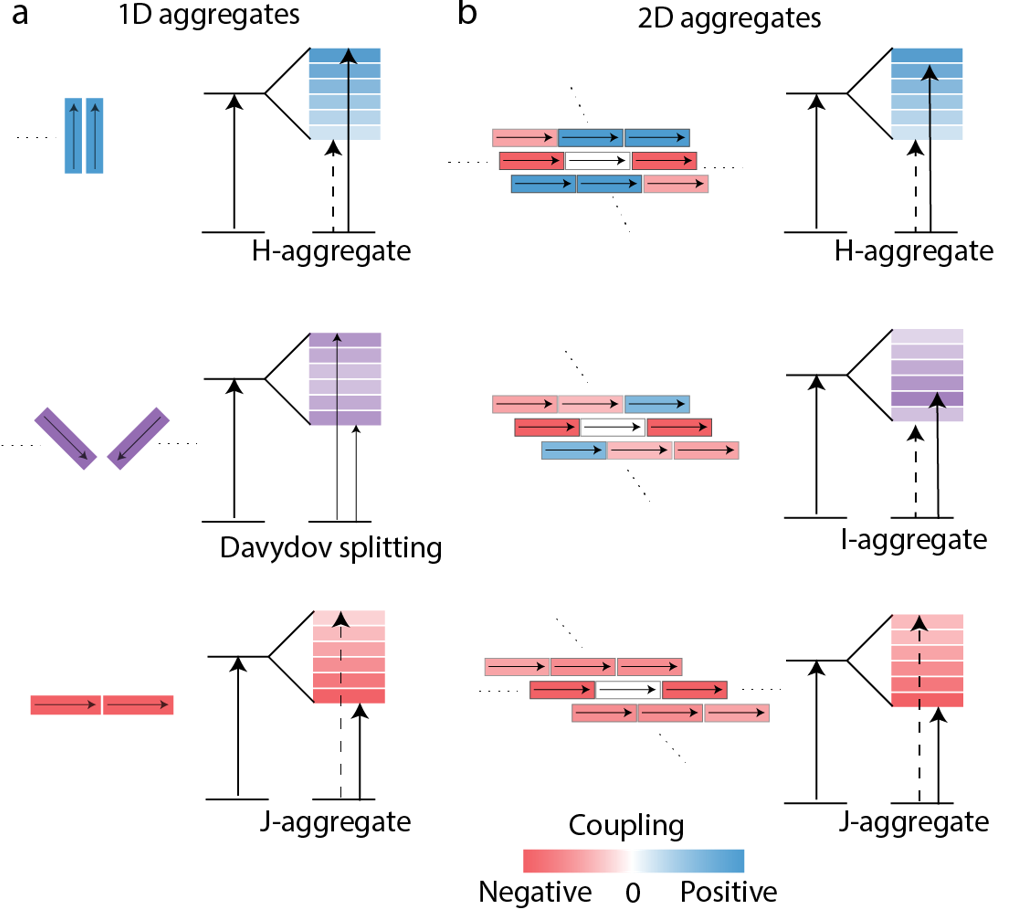
Classification Schemes for 2D Molecular aggregates
Life uses ordered repeat units to create mesoscale structures which can conduct the flow of energy. In plants and bacterial, these structures can absorb light and move it coherently through strongly coupled transition dipole moments. We are developing synthetic analogs to these structures, understanding how simple building blocks lead to complex excitonic structure and dynamics.